- Home
- > Project Serialization
Track and Trace on Pharmaceutical packaging lines fulfils FDA compliance
This international pharmaceutical company implemented serialization on multiple packaging lines. This enabled the application of unique and verifiable product identifiers to individual boxes, cases and pallets of drug products.
Serialization lines use high speed printers and cameras to print, apply and verify these unique product identifiers.

Each level of the process has separate identifiers and gets associated with each container that it is placed within. Several identified bottles may be wrapped together into a bundle and given an identifier, then placed into a case with its own identifier, and again onto a pallet.
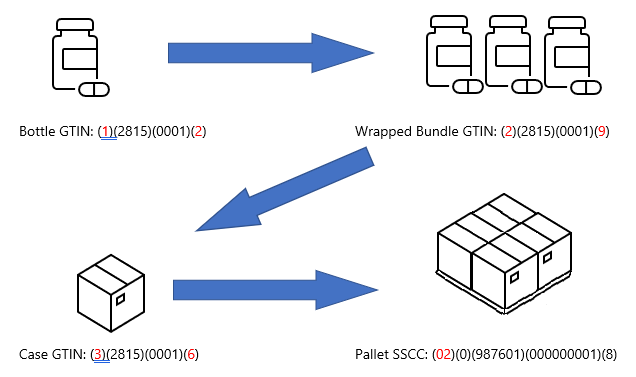
The overall goal is so that these items can be tracked throughout the distribution process. A pharmacist can verify this information when dispensing the product to a patient, creating a traceable connection from where and when the product was packaged to how it was shipped to the pharmacist.

This system used the GS1 global standard, using GTINs with serial numbers to derive a truly unique identifier for each item.
GCS provided engineering and project management for the design, integration and implementation of the project. GCS also participated in 7 Factory Acceptance Tests (FAT) representing the interests of the customer and verifying that the equipment met the customer requirements.
GCS performed the following tasks:
- Project Serialization Lead
- Hardware design, implementation, and integration of all associated serialization equipment into existing packaging lines.
- Developed PLC, HMI, and associated equipment custom software to reliably integrate all serialization equipment into the existing packaging lines.
- Integration of user security with customers’ existing Active Directory for authentication and user rights.
- Developed and assisted in the development of cGMP documentation for labelers, tray packers, case packers, serialization equipment, and more:
- Engineering Studies
- User Requirements Specification (URS)
- Functional Requirements Specification (FRS)
- Site Acceptance Tests (SAT)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Integrated Line Qualification (LIQ)
- SOPs, Protocols, Work Instructions, Reports and additional Specifications
- Change Control, Impact Assessments, Risk Analysis

QUANTIFIABLE RESULTS
Fully serialized pharmaceutical production lines.
Plant personnel can access serialization data, generate on-demand reports and track their products.
Full compliance with FDA mandate and requirments.
